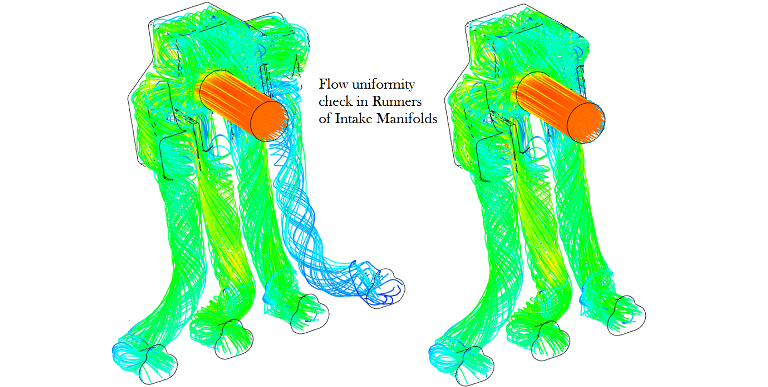
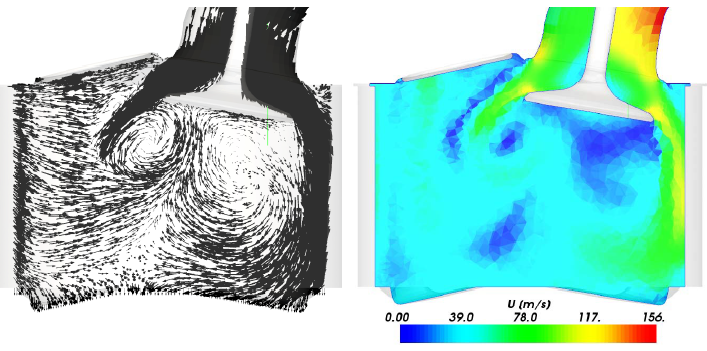
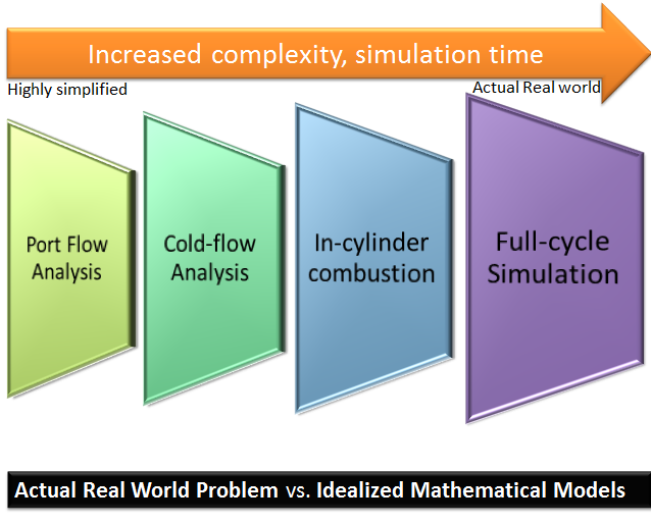
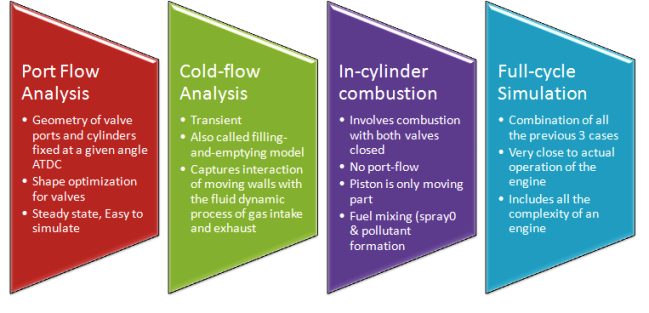
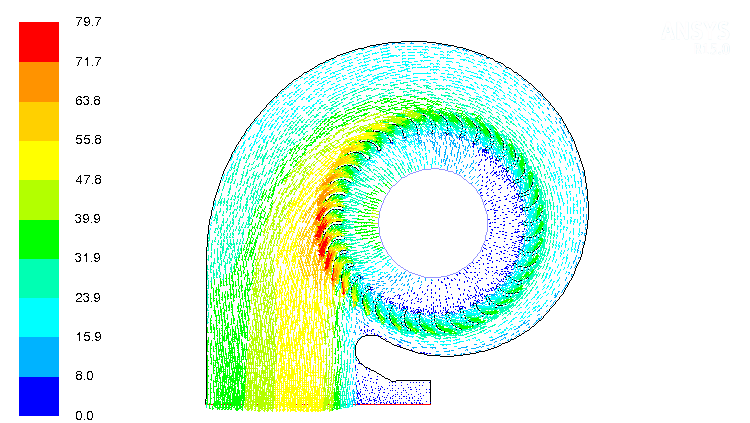
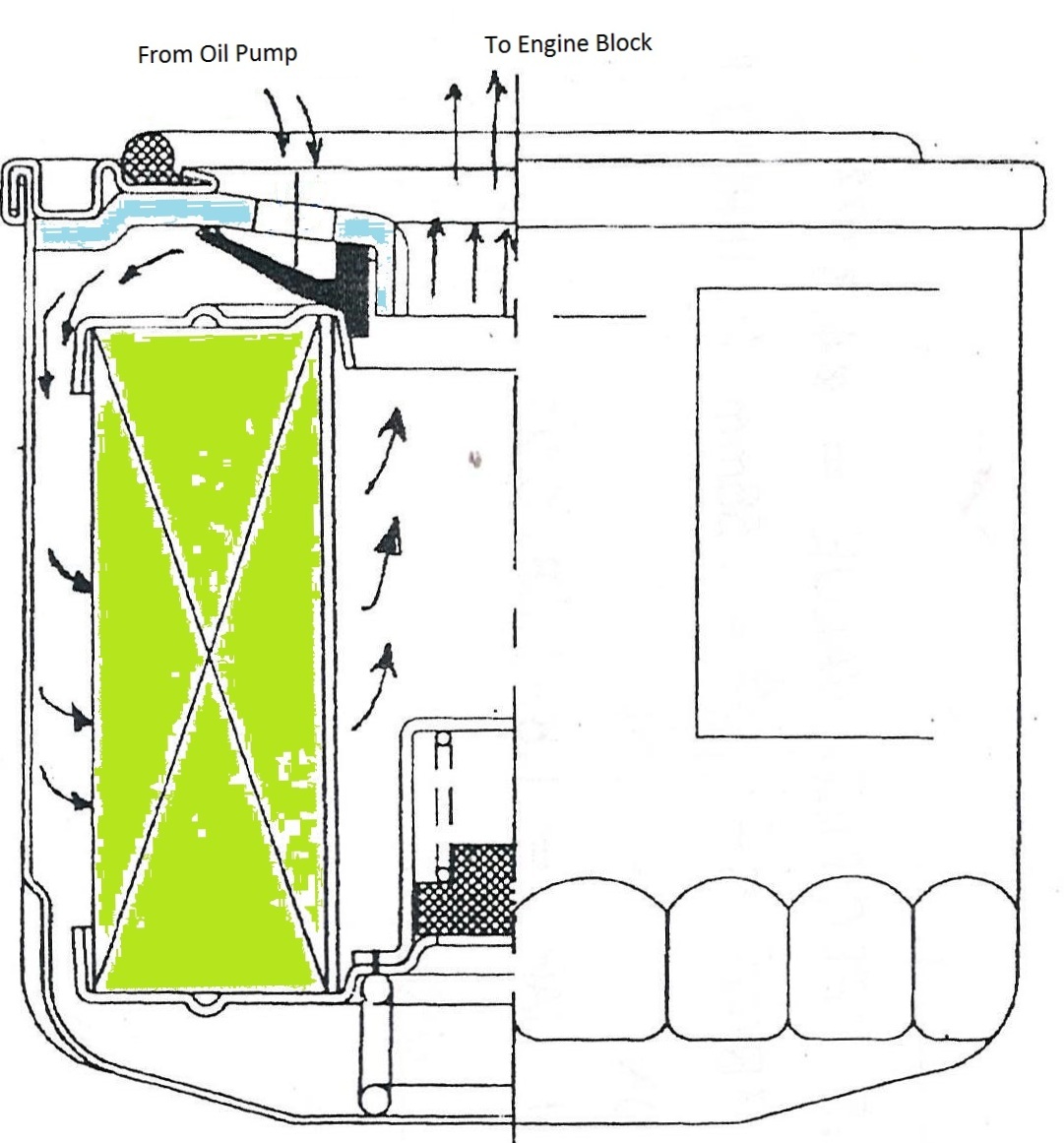
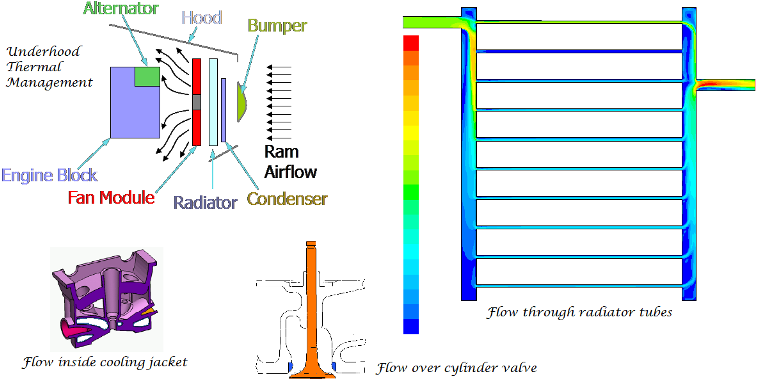
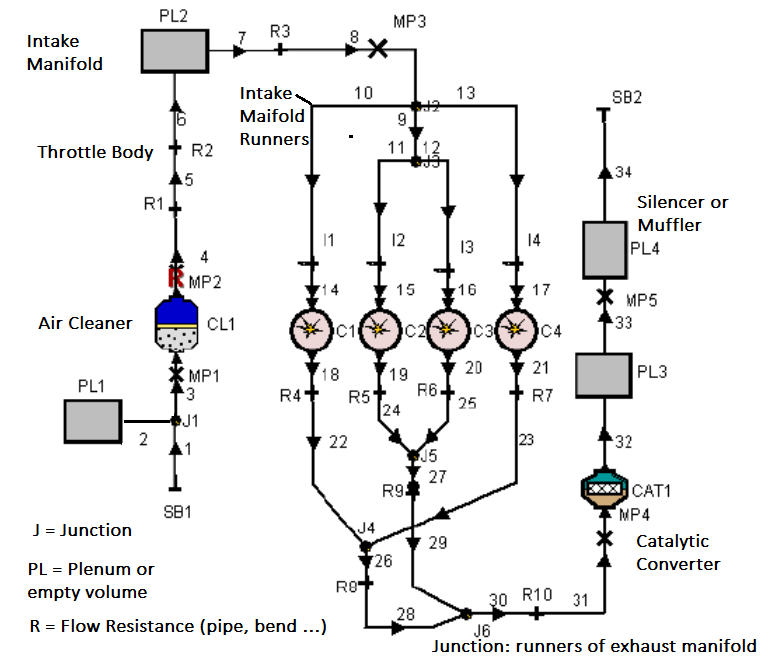
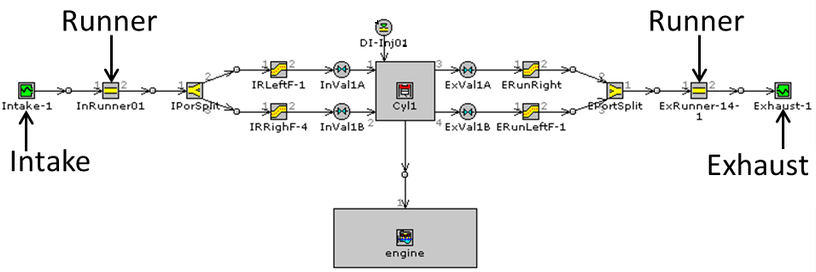
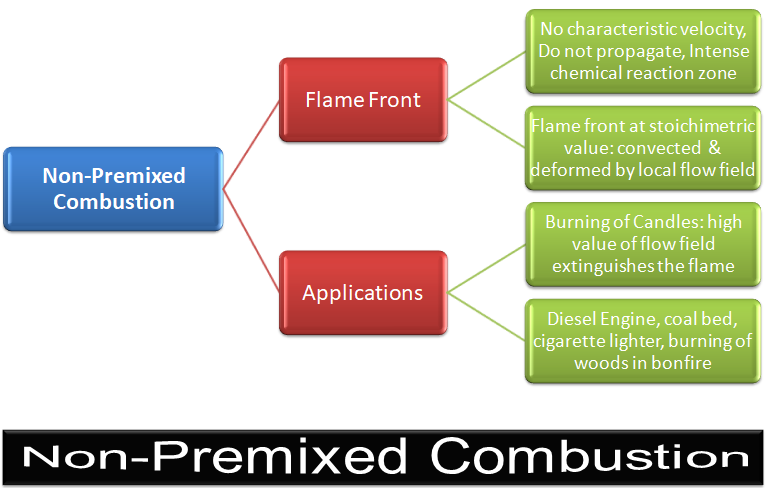
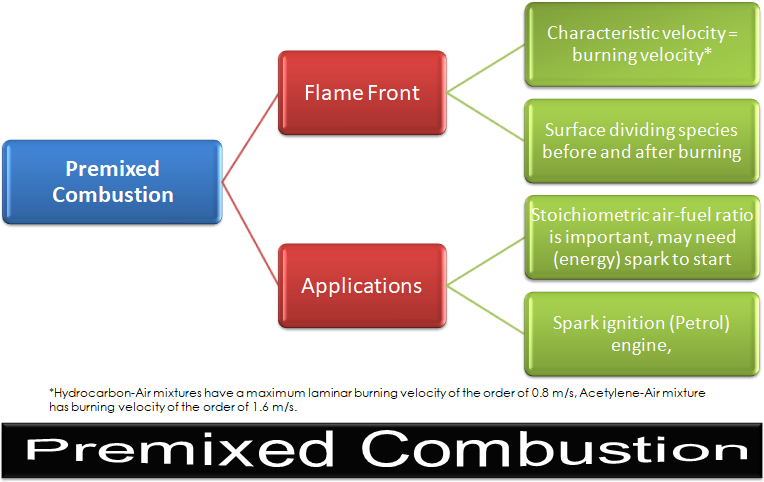
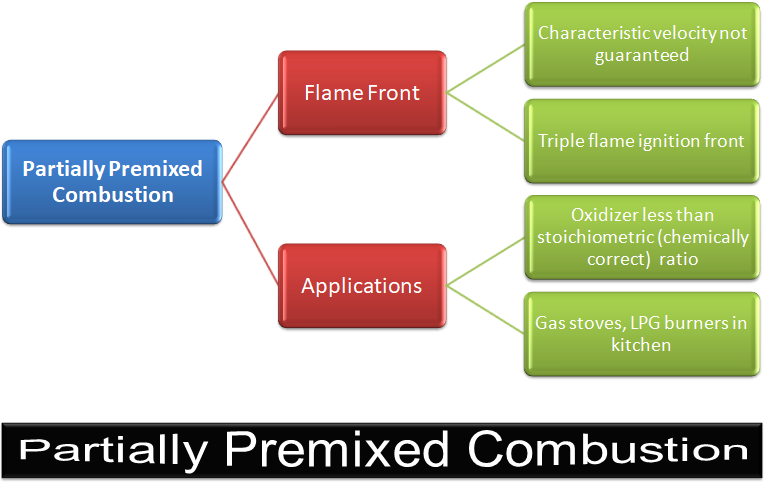
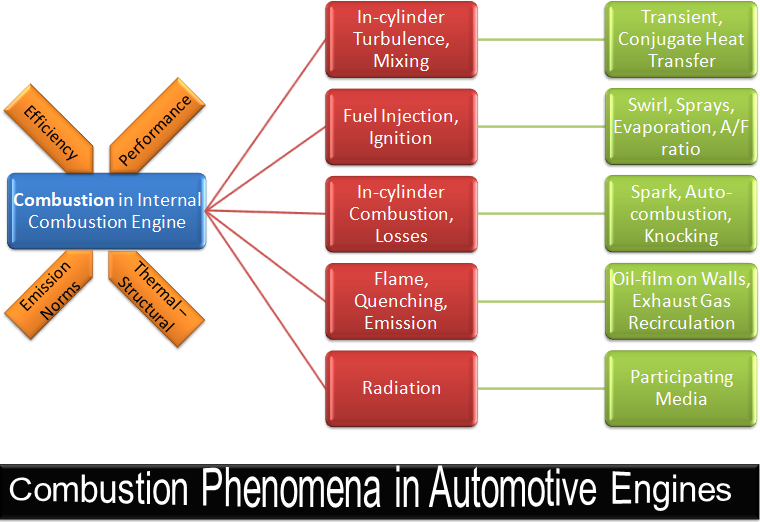
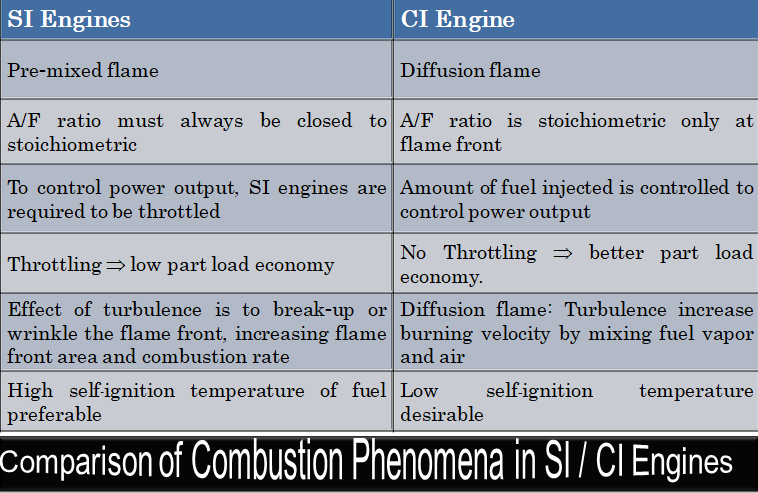
CFD simulations have wide ranging applications in automotive field including simple flow in intake manifolds to most complex phenomena of combustion. More applications include underhood thermal management, loss coefficient calculations for intake and exhaust ports, front-end flow, engine cooling jacket flow optimization, flow distribution in radiator tubes, external aerodynamics, de-icing / hot-soak-down / cold-soak-down / thermal comfort in HVAC, EGR coolers, flow uniformity check for catalytic converters ... Apart from standalone utilities, the detailed 3D simulation models can be coupled with 1D lumped simulation programs such as GT-Power and AmeSim.
Table of contents: Battery Cell Performance Estimation | Defrost and Demist Simulations | Car Roll-over Calculation | Slider Crank Mechanism [+] EV: Electric Vehicle and Battery PacksTypical SI and Diesel operating value comparisons
| Parameter | SI | Diesel |
| BMEP - Naturally aspirated | 10-15 [bar] | 10 [bar] |
| BMEP - Turbocharged | 15-25 [bar] | 15-25 [bar] |
| Power density - Naturally aspirated | 50-70 [kW/L] | 20 [kW/L] |
| Power density - Turbocharged | 70-120 [kW/L] | 40-70 [kW/L] |
| Fuel | ||
| H to C ratio | CH1.87 | CH1.80 |
| Stoichiometric A/F | 14.6 | 14.5 |
| Density | 0.75 [g/cc] | 0.81 [g/cc] |
| Lower Heating Value (mass basis) | 44 [MJ/kg] | 43 [MJ/kg] |
| Lower Heating Value (volume basis) | 3.30 [MJ/L] | 3.48 [MJ/L] - (5.5% higher) |
| Lower Heating Value (CO2 basis) | 13.9 [MJ/kg-CO2] | 13.6 [MJ/kgCO2] - (2.2% lower) |
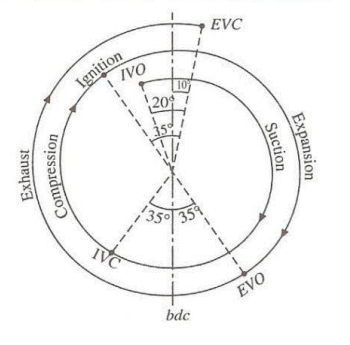
A petrol engine develops vacuum in intake manifold due to throttling (ventury effect). Diesel engines do not have throttle valve (the accelerator pedal is used to control fuel injection directly) and hence vacuum created in intake manifold is only due to pressure drop in air cleaner and intake ducts. SAE Paper 2011-01-0071: "Performance Optimization of Vacuum Pump for a Diesel Engine for Euro V Application by Experiments" - "Vacuum pump is a device which gets the drive from engine cam shaft. In some designs, it is driven by the alternator shaft. The main function of vacuum pump is to evacuate the air from the brake booster tank, thus creating vacuum, which can be used for brake application. In addition to this, in new generation engines, to meet the Euro V emission targets, vacuum pump also has to create vacuum in the auxiliary tank which will be used to actuate the turbo charger waste gate actuation mechanism and EGR valve. Article "Experimental Study of the Effect of Air Filter Pressure Drop on Internal Combustion Engine Performance" by Tadeusz Dziubak and Miroslaw Karczewski: "When operating at rated conditions, passenger car engines draw 150–400 [m3/h] of air per hour. For truck engines, this value is 900–2000 [m3/h]."
As per "summitracing.com/knowledgebase/article/SR-05306/en-us": Engine Function - Vacuum Range: Cranking/3-5 in-Hg, Idle/14-20 in-Hg, Cruising/10-15 in-Hg, Deceleration/21-25 in-Hg. Note that 1 in-Hg = 3386.4 [Pa].Electrical Vehicle vs ICE Vehicle
Electrical vehicles are the new technology emerging (with potential and willingness) to replace fossil fuel based internal combustion engine (ICE) driven vehicles. A short and preliminary comparison of the two technology can be summarized as follows.
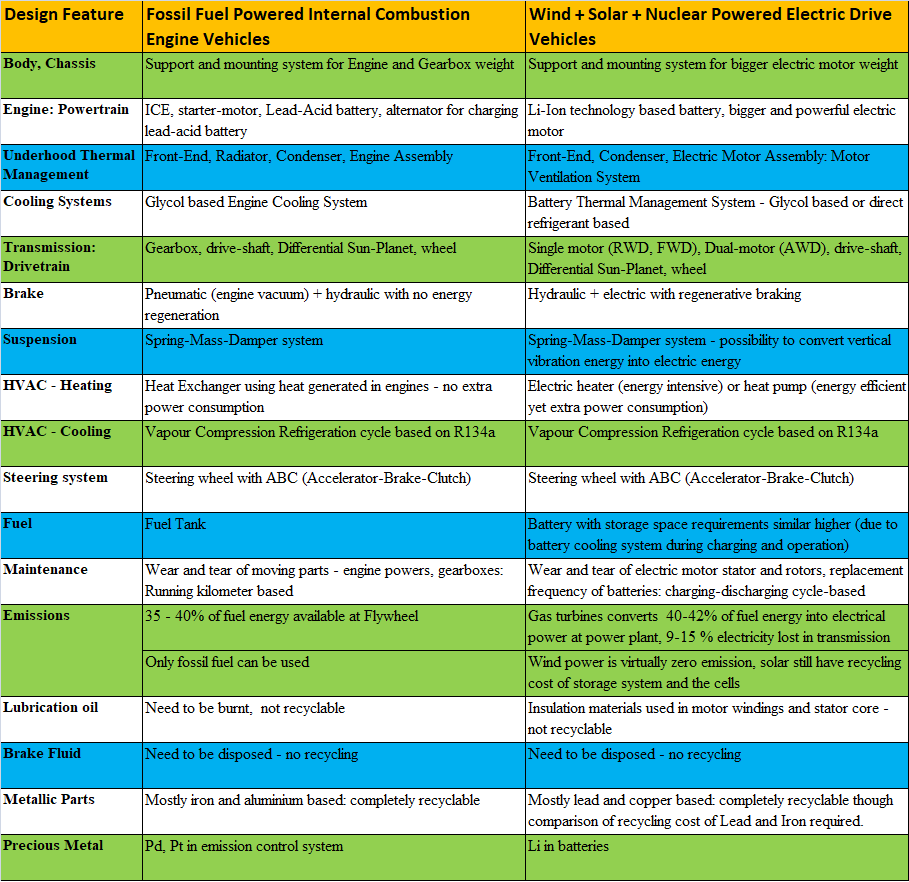
- Cooling of electric drive motors: the hot gas can be used in a gas-to-gas heat exchanger for heating of passenger cabin.
- Thermal comfort in cabin - same as conventional internal combustion engine based vehicle.
- Battery Thermal Management System: needs to be heated under cold condition and cooled under hot conditions.
- Cooling of brakes - same as conventional vehicle, regenerating braking can be used as electrical circuits are present everywhere. Brake force still hydraulic but actuation can be electrical.
EV: Electric Vehicle and Battery Packs
EDV: Electric-Driven Vehicle, HEV: Hybrid Electric Vehicle - the hybrid vehicle battery continues to release and store large amounts of energy while the car accelerates or decelerates. The idea is to let the combustion engine run near maximum efficiency point always and the shortfall or excess of energy is provided by or stored in the battery.As per "Battery Guidance Document - Transport of Lithium Metal, Lithium Ion and Sodium Ion Batteries" by IATA: Cell means a single encased electrochemical unit (one positive and one negative electrode) which exhibits a voltage differential across its two terminals. Battery means two or more cells or batteries which are electrically connected together and fitted with devices necessary for use, for example, case, terminals, marking and protective devices. Units which have two or more cells that are commonly referred to as "battery packs", "modules" or "battery assemblies" having the primary function of providing a source of power to another piece of equipment.
- LIB: Lithium-ion batteries, BESS: Battery Energy Storage System, BTMS: Battery Thermal Management Systems, ECM: Equivalent Circuit Modelling
- Cell: a single functional battery (cathode and anode)
- Battery Module: a standalone set of batteries or cells stacked and connected internally in series-parallel manner and acts as one unit. E.g. a battery module of 8 cells shall have only one connection to utilize all 8 cells simultaneously.
- Battery Pack: It is battery modules stacked and connected externally with one another. At any point of operations, either all the battery modules can get activated or only pre-defined few.
- PEEM: Power Electronics and Electric Motor, EES: Energy Storage System (the battery pack), CFL: Combined Flow Loop
- FEHX: Front End Heat Exchangers (Radiator, condenser, oil cooler)
- OCV: Open Circuit Voltage, SOC: State of Charge, DOD: Depth of discharge: percentage of the battery that has been discharged relative to the overall capacity of the battery
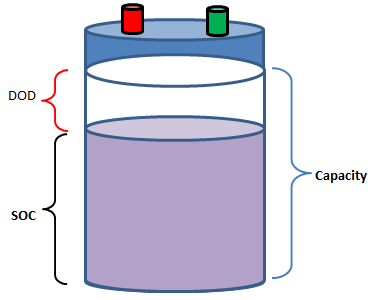
- Life of Battery: Storage life - number of calendar days after which SOC is reduced to 50%, Cycle Life - number of charging-discharging cycle after which is becomes non-functional. A battery may have 15,000 cycles at a DoD of 50% but only 3,000 cycles at 95% DoD.
- The C-rate is a measure of the rate at which a battery is being discharged or charged relative to it is maximum capacity. Batteries are typically rated at 1C which means that a 10 [Ah] battery would provide 1 [A] for 10 [hours] if discharged at 1C rate.
- Storage Capacity: Specific energy - [W.h/kg], Energy density -[W.h/L], Heat released in thermal runaway - [W.h/cell]
- Thermal Runaway (TR): Thermal failure of individual cells could be initiated for different reasons such as internal short-circuit, over-heating and over-charging or discharging. These further causes increase in cell temperature and trigger chemical reactions. Subsequently highly exothermic reactions result in a rapid self-heating of the cell including the nearby cells. TR describes a situation when a cell spontaneously self-destructs due to temperature increase.
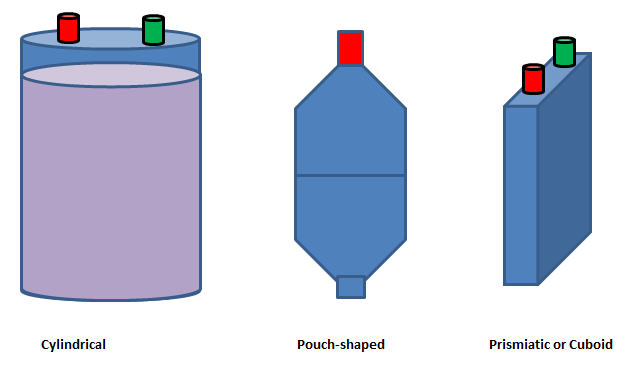
- Batteries (cells) are typically available in cylindrical, pouch and prismatic (cuboid) shapes
| Discharging (acutal operation cycle) | Charging (energy storage cycle) |
| During this cycle, lithium ions form from the ionization of lithium atoms in the anode. | The lithium ions return to the anode from the cathode and electrons are transferred from the anode to the cathode. |
| Oxidation reaction takes place at anode | Reduction takes place at the cathode |
| The lithium ions move from the anode and pass through the electrolyte until they reach the cathode, where they recombine with their electrons and electrically neutralise | Lithium ions flow from the cathode to the anode, and electrons flow from the anode to the cathode |
| The lithium ions are small enough to be able to move through a micro-permeable separator between the anode and cathode | |
Construction and Bill-of-Material
Outer Shell (battery / containment rigid case), Can (inner flexible shell), Jelly or Active Material, Cathode and Anode (Cathode: Typically aluminium foil, Anode: Typically copper foil), Current Collector Tabs, Binders, Lid, Terminal, Electrolyte (LiPF6 salt), Separator (thin microporous polymer membrane). The terminal is welded to the current collector tabs, and the lid is welded to the battery case. In a battery pack, bus-bars are welded to the terminal to obtain desired series-parallel connection of the cells.Functional parts of the battery: anode, cathode, separator, and electrolyte. Packaging materials: Pouches, Cases, Cans, Tabs. Cathode Active Materials: LFP, LCO, LMO, NMC, NCA, CSG. Anode Active Materials: Graphite, Graphene, LTO. Anode Foils: Copper, Nickel. Cathode Foils: Aluminium. Cell Casing encloses the anode, cathode, and separator where a polypropylene resin pouch is used to enclose these components before they are placed in the aluminium casing. Current collectors provide a path for electrons to flow to and from the external circuit. They are essential for the battery's electrical function, but they do not store energy.
Anode and cathode are physically separated by a electrolyte-filled separator which allows movement of positive ions through it. Thus, separator resists internal short circuits, and facilitates the movement of ions between electrodes.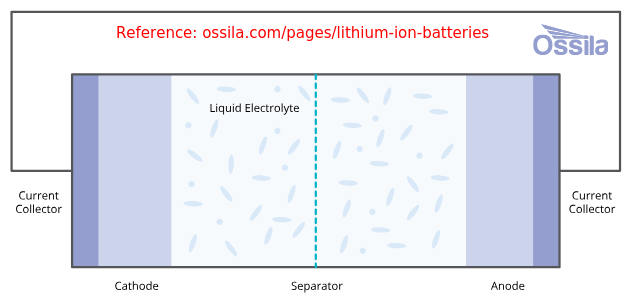
Terminals are the external points of connection for the device being powered or the charger, located on either end of the battery. They are connected to the internal current collectors, usually with metal tabs (such as aluminium tab for anode 'negative' terminal, nickel or copper tab for the cathode 'positive' terminal) that pass through the lid or casing. Legacy batteries typically have 2 terminals (1 at the cathode and 1 at the anode), more recent batteries can have far more than 2 terminals. Tabs are the internal components that connect the current collectors to these terminals. Binders: Polymers like polyvinylidene difluoride (PVDF) improve the structural integrity of the electrodes and adhesion to the current collectors.
Reference: ul.org/researchelectrochemical-safetygetting-started-electrochemical-safetywhat-causes-thermal: "Thermal runaway is a phenomenon in which the lithium-ion cell enters an uncontrollable, self-heating state. Thermal runaway can result in extremely high temperatures, violent cell venting, smoke and fire." Reference: A Review of Lithium-Ion Battery Thermal Runaway Modelling and Diagnosis Approaches by Manh-Kien Tran et. al. "The uncontrollable and irreversible nature of thermal runaway is the main challenge for the mitigation of Li-ion battery safety hazards. "
Sample data for a battery
| SM Bexel battery manufacturer, Gangseo-gu, Seoul, South Korea* | |
| Nominal voltage (V) | 3.75 |
| Nominal capacity (Ah) | 52.3 |
| Negative electrode | Copper-Graphite |
| Positive electrode | Aluminum-NCM523 |
| Electrolyte | Polyethylene |
| Electrical conductivity σ (S/m) | 3.77 × 10 |
| Electrical conductivity σ (S/m) | 5.96 × 10 |
| Thermal conductivity (W/m-K) | (x, y, z) = (25.5, 25.5, 0.794) |
| Specific heat (J/kg-K) | 566 |
| Density (kg/m3) | 2695 |
Schematic and Arrangment of Cells in a Battery Pack: The arrangement of cells inside a battery pack can have different combination of series-parallel connection electrically. In terms of physical layout, they can be stacked in staggered or parallel arrangement.
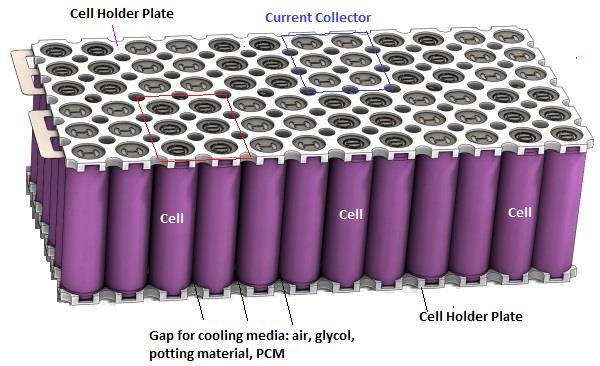
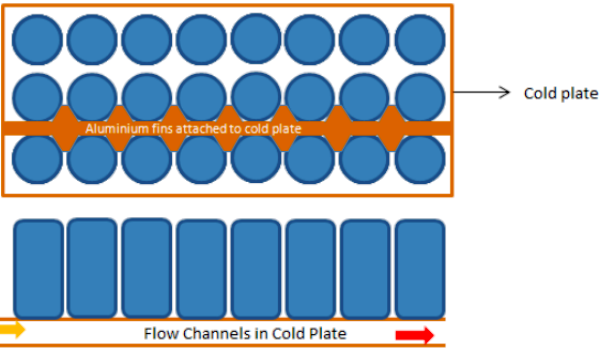
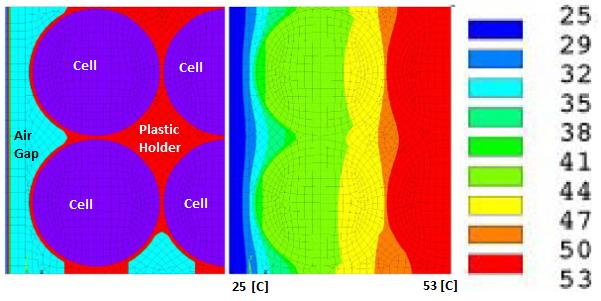
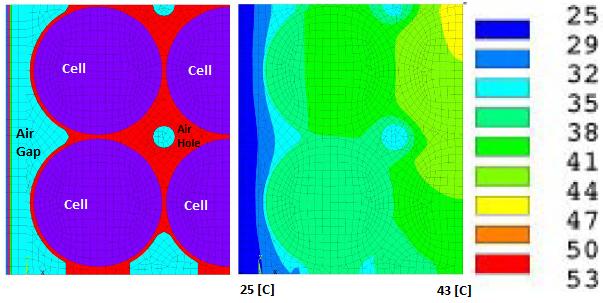
Battery Cell Performance Estimation
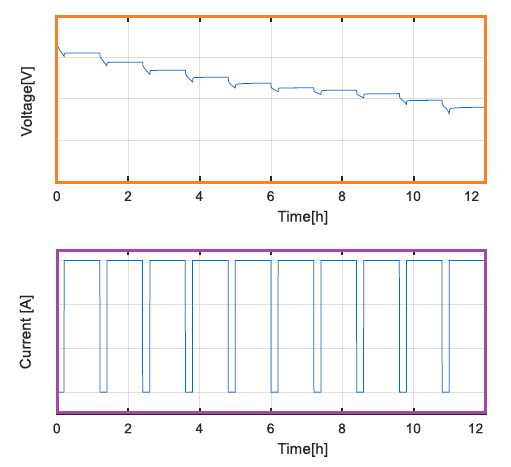
Equivalent Circuit Model (ECM): The parameters for ECM is usually estimated using two experiments, the pulse test and the low current test. This model requires few parameters such as open circuit voltage, ohmic resistance and current pulse. Lithium Iron Phosphate (LFP), Lithium Nickel Manganese Cobalt Oxide (NMC) and Lithium Nickel Cobalt Aluminum Oxide (NCA) are the most commonly used cell chemistries.In order to estimate the parameters, two tests are performed: (a)the pulse test and (b)low current experiment. The purpose or low current test is to estimate the Open Circuit Voltage (OCV) accurately. The aim of the pulse test is to get OCV at different SOC. Data generated in both these tests can be used to generate parameters of ECM described below
ECM MODEL
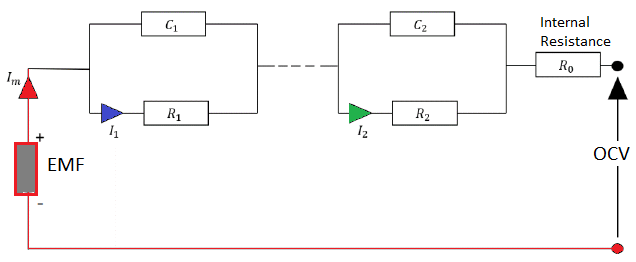
PULSE TEST
Electrochemical Model: The model is based on the electrochemical processes that occurs inside the cell/battery. The detailed process phenomena of inner cell is simulated using the chemical characteristics and design parameters. The electrochemical models use complex non-linear differential equations to describe the battery internal dynamic characters with many unknown parameters and uses thermodynamics and electrochemical kinetics equations.
Battery Thermal Models
Heat generated in the lithium-ion battery is mainly from two processes: entropic heat generation due to changes in electrochemical reactions (function of the temperature gradient of open-circuit voltage dE/dT of the cell), and ohmic heating (approx. 54% of total heat) resulting from current flow through cell internal resistance during charge and discharge process. The cell heat generation rate depends on a number of factors such as C-rate, the temperature of the cell, SOC whether the cell is charging or discharging. Cells have higher heat generation at low temperature as well when the C-rate is high. However, the heat generate rate is lower at higher cell temperature due to lower electrical resistances at higher temperature. 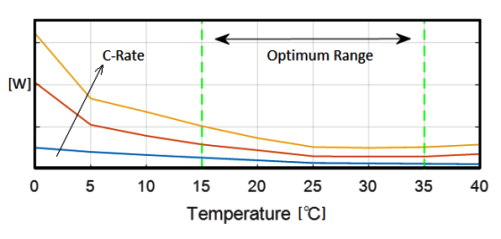
Lumped capacitance thermal model: The model splits the battery core and battery case as two sperate isothermal nodes, all components inside the core such as anode, cathode, active material... are assumed to be a single homogenous material with masss-weighted or volume-weighted averaged properties.
- Inputs: heat generation rate [W], thermal resistance or conductivity [W/m-K] and specific heat capacity [J/kg-K] of the cell
- Output: mean temperature inside cell and the casing
- Method: one-dimensional thermal network
- Tool/program: MATLAB/Simulink, MS-Excel, AMESIM...
MSMD: Multi-Scale Multi-Domain Model:
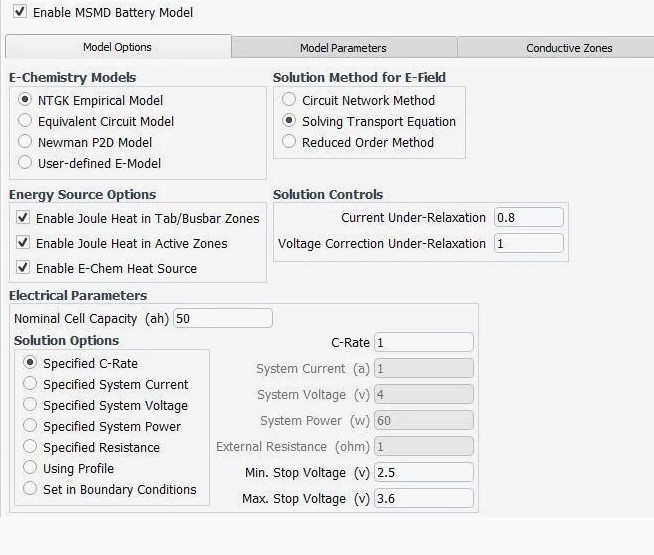
NTGK Empirical Model: Newman, Tiedemann, Gu, and Kim (NTGK) Model - thermal abuse behavior of lithium-ion batteries - a semi-empirical but integrated thermal–electrochemical coupled transient analysis approach as the method relies on curve fitting the experimental data. The empirical parameter designated as U and Y are modelled as polynomial function of DOD (Depth of Discharge): U = a0 + a1 x DOD + a2 x DOD2 + a0 x DOD3 + a0 x DOD4 + a0 x DOD5, Y = b0 + b1 x DOD + b2 x DOD2 + b3 x DOD3 + b4 x DOD4 + b5 x DOD5. These fitting parameters are used to determine the potential and current density distribution on the electrodes during discharge.
Finite element analysis battery thermal model: The model describes the battery heat transfer in three-dimensional. This model is more accurate and useful to study temperature gradients in the cell and surrounding. However, the model requires a large number of parameters and data which needs to be determined through theoretical / empirical calculations or experiments.Thermal Management of EV
Thermal management entails regulating heat flows into the cabin of the vehicle and out to the ambient from hot parts. The goal is to ensure components operate in their respective temperature limits while providing comfortable temperatures for passengers in the vehicle interior.- Thermal management systems in electric vehicles are generally more complex than in conventional vehicles featuring combustion engines.
- Thermal protection for hundreds of components real-time is a complex electro-mechanical and mechatronics process.
- The battery needs to be cooled or heated depending on the ambient and operating conditions.
- Waste heat is not available from a combustion engine to heat the vehicle interior. This necessitates the use of energy-efficient measures heat pump instead of direct electric heaters. Normally a PTC (Positive Temperature Coefficient) heater provides heating in cold weathers.
- The refrigerant circuit and the cooling circuit needs be optimally synchronized to transfer heat inside the vehicle. Interconnection of these two different fluid circuits changes depending on the (cabin/battery pack) heating or cooling requirements. The refrigeration loop has compressor, condenser, receiver-drier, expansion valves (one each for chiller and evaporator), chiller and evaporator. The chiller is used to cool the coolant in hot weather when the radiator alone is not sufficient.
- Coupled use of 1D system simulation to 3D thermal solutions can help reduce chance of detection of thermal issues late in the design cycle.
Hazardous gas vent in Battery Packs
Thermal runaway of Li-ion batteries is the phenomenon of exothermic chain reactions within the battery. If a condition of thermal runaway occurs in battery pack, there is a likelihood that gases vented from the affected cells could ignite and combust as the gases generated are highly flammable. Understanding how a battery vents, and extreme case the severity of the resulting combustion is key to improving the safety of the battery pack. In simulations to model combustion phenomena, a battery cell is assumed to have entered thermal runaway and begins to vent out gas products. A short-circuit spark near the faulty cell can be simulate to ignites the gases. Because most of the battery packs are stuffed with solids and little volume of air voids are present, there is limited oxygen available within the battery pack, most of the combustion occurs outside of the packs. The uneven temperature can easily cause local polarization in the battery, explosion risk increases with increasing discharge rates.
Thermal runaway occurs from various forms of mechanical (destructive deformation), electrical (exposure to water, conductor contamination, electric shock...), and thermal abuse (High- and low-temperature environments, multiple overdischarges followed by charge) or operations beyond permissible boundaries. As the separator between the anode and the cathode either collapses, tears down, or is pierced, an internal short-circuit of the battery generates high amount of heat, which in turn increase the rate of electrochemical reactions causing excessive heat generation. As per H. Maleki, J.N. Howard, "Internal short circuit in Li-ion cells", within the first minute of Internal Short-Circuit, 70 % of the energy can get released.
"Experimental study on thermal runaway and vented gases of lithium-ion cells" by L. Yuan et al
| Summary of vented gas characterization | |||||||
| Type | H2 (%) | CO (%) | CO2 (%) | CH4 (%) | C2H2 (%) | C2H4 (%) | C2H6 (%) |
| LFP: LiFePO4 | 23.34 | 4.50 | 25.39 | 5.90 | 0.08 | 3.26 | 1.29 |
| LTO: Lithium titanate | 8.41 | 5.30 | 37.6 | 1.23 | 0.0008 | 1.38 | 0.40 |
| NMC 1: Lithium nickel manganese cobalt | 12.39 | 30.3 | 13.22 | 10.5 | 0.0026 | 0.10 | 0.16 |
| NMC 2: Lithium nickel manganese cobalt | 12.54 | 28.06 | 19.91 | 12.9 | 0.0027 | 0.16 | 0.21 |
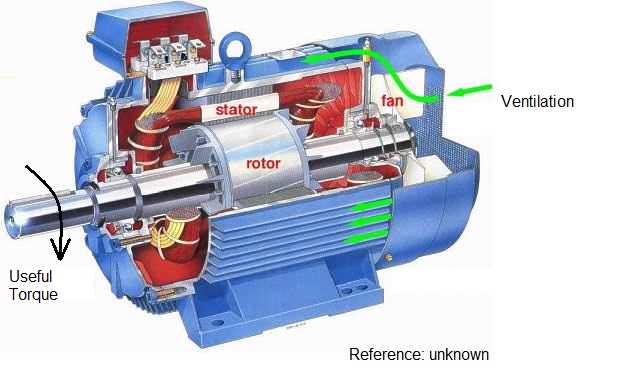
Ventilation of an Electric Motor
Recyclability of Battery Component - LAB (Lead Acid Batteries)
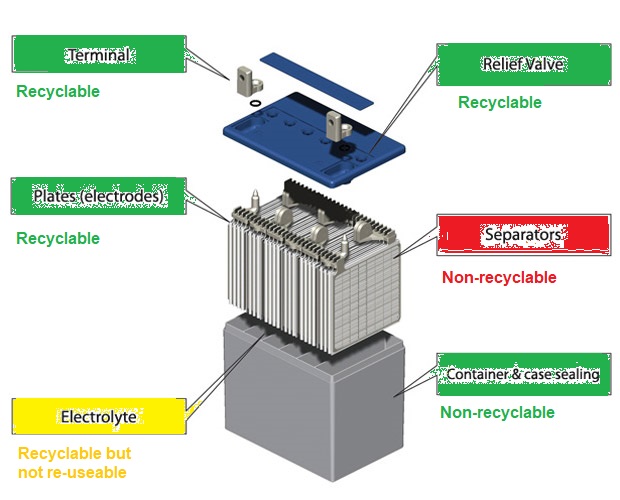
Recyclability of Battery Component - LIB (Li-Ion Batteries)
As of now, no known and scalable (technically as well as commercially) method exists. The cells used in mobile phones are still being dumped as solid waste. There is no constituency for holistic, cradle-to-grave view of energy production with least total environmental impact! Total "cradle to grave" CO2 emissions ~ same for all propulsion methods and energy sources! - Reference: www.hydrogen.energy.gov/pdfs/14006_cradle_to_grave_analysis.pdf
Automotive Parts and Sub-assemblies
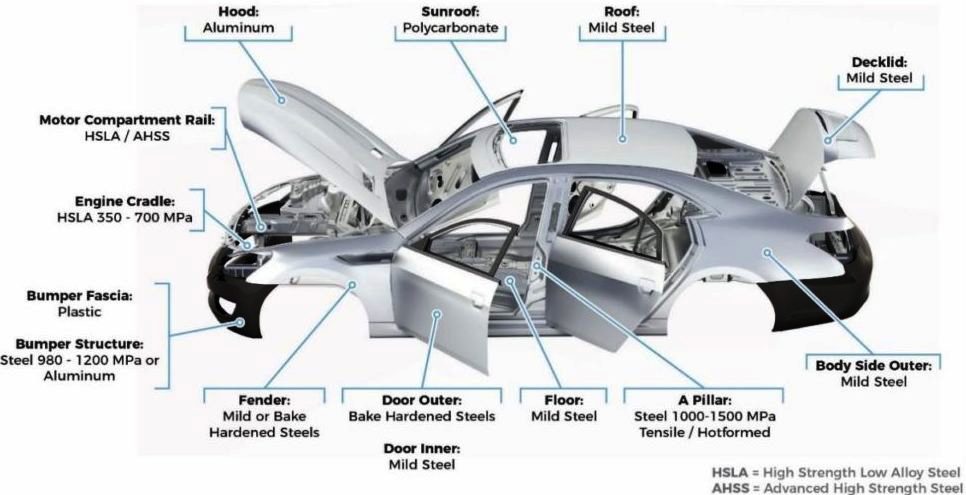
Application of Numerical Simulations
Automotive
There are wide range of applications of numerical methods such as CFD in automotive domain ranging from system level External and Underhood aerodynamics to brake cooling to the simulation of combustion phenomena inside engine cylinders. At the same time, the implementation of 3D simulation with 1D tools such as AVL-Boost, Ricardo-Wave and GT-Power suites significantly enhances the capabilities of each other. […]
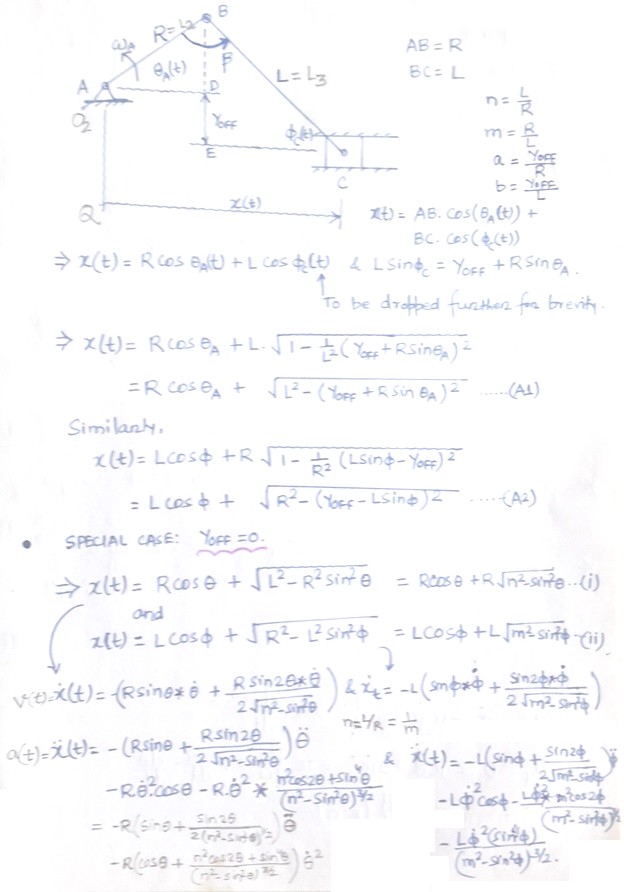
CFD can be used to iterate the designs without actual prototyping. For example, the flow uniformity though the various runners of an intake manifold can be ensured at the early stage of development.